Learn About Breakthrough, Fully Implanted Hearing Devices Designed With The Patient’s Quality of Life in Mind
Envoy Medical is a hearing health company focused on creating breakthrough, fully implanted hearing device solutions for people living with moderate to profound hearing loss. Our Esteem® fully implanted middle ear implants (FI-AMEI) and Investigational Fully Implanted Acclaim® Cochlear Implants* offer significant technological differentiation from competitive hearing device manufacturers, using the natural ear to pick up sound without relying on artificial microphones—similar to the way nature designed and intended.
Envoy Medical is located in Minnesota and is the only American-based hearing implant company currently listed on a major U.S. stock exchange. Join us as we develop future-forward solutions for people living with hearing loss.
JOIN US:

1K
Patients wearing Esteem® implant devices
Global
~$84B+
Untapped potential cochlear implant addressable U.S. market opportunity
U.S.
35
Patents issued, 13 patents pending
U.S.
3-5%
Current market share goal by 2030
Global
~$45M
Gross profit (65-70% gross margin) by 2030
JOIN US:
Our Hearing Products
Fully Implanted Acclaim® Cochlear Implant*
The Fully Implanted Acclaim® Cochlear Implant* is positioned to become the first-of-its-kind, fully implanted cochlear implant for people with severe to profound hearing loss. It is currently an investigational device being studied in clinical trials and received a Breakthrough Device Designation in 2019 by the FDA.
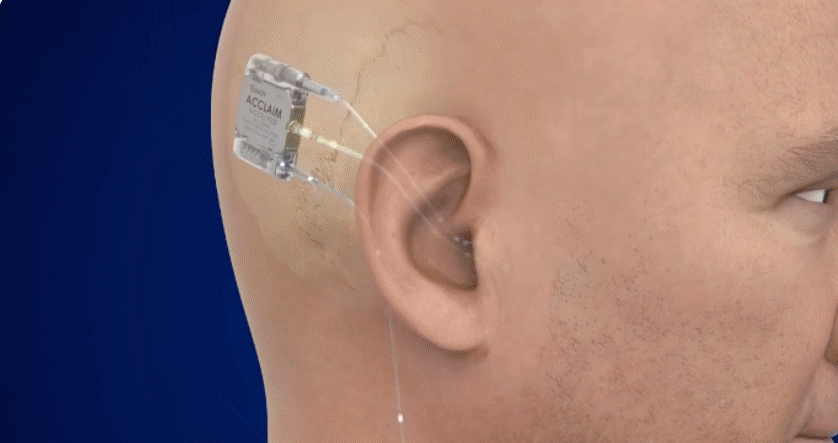

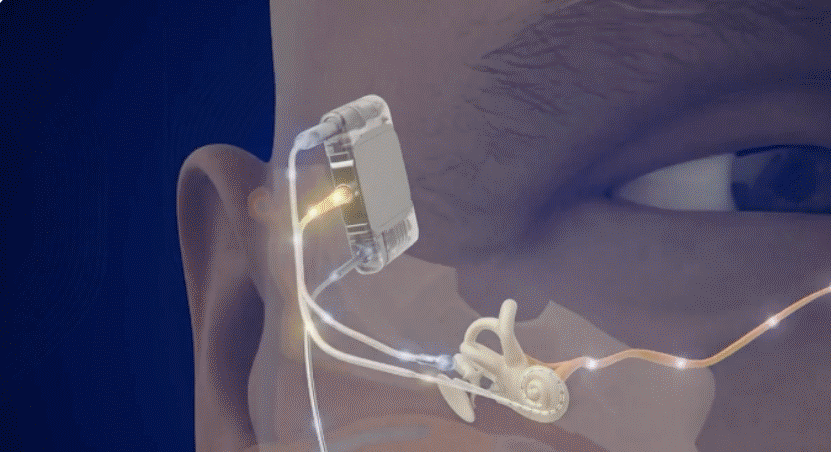
For illustrative purposes only; may not be indicative of individual characteristics, size placement, functionality, or use
Key features of the Fully Implanted Acclaim® Cochlear Implant* include:
Discreetly internal with no need for an external microphone, designed to use the ear to pick up sound.
Dependable design, allowing for true 24/7 hearing and for use in many environments and activities.
Easy to wear with no expensive external sound processors to replace when lost or damaged, no frequent battery changes or charging and a rechargeable battery that’s expected to last several days between charges and is designed to last 8-12 years.
Magnet-free, designed to be MRI compatible.1
*MRI testing and compatibility not yet determined.
Esteem
FI-AMEI
Esteem is a revolutionary, fully implanted active middle ear implant for people with moderate to severe hearing loss. It is the first and only FDA-approved, fully-implanted active middle ear implant (FI-AMEI) hearing device that enables wearers to have nothing in their ear but sound—allowing them to be fully connected and engaged in life in ways they never thought possible.
Esteem has already been implanted in approximately 1,000 people and has the potential to increase market share following the reintroduction of the Hearing Device Clarification Act, which would allow Medicare coverage as Esteem would be reclassified as a prosthetic device, not a hearing aid. Significant progress is already being made to obtain reimbursement for a previously un-reimbursed device. If reimbursement were to change, market size could become similar to the size of the cochlear implant market over time.
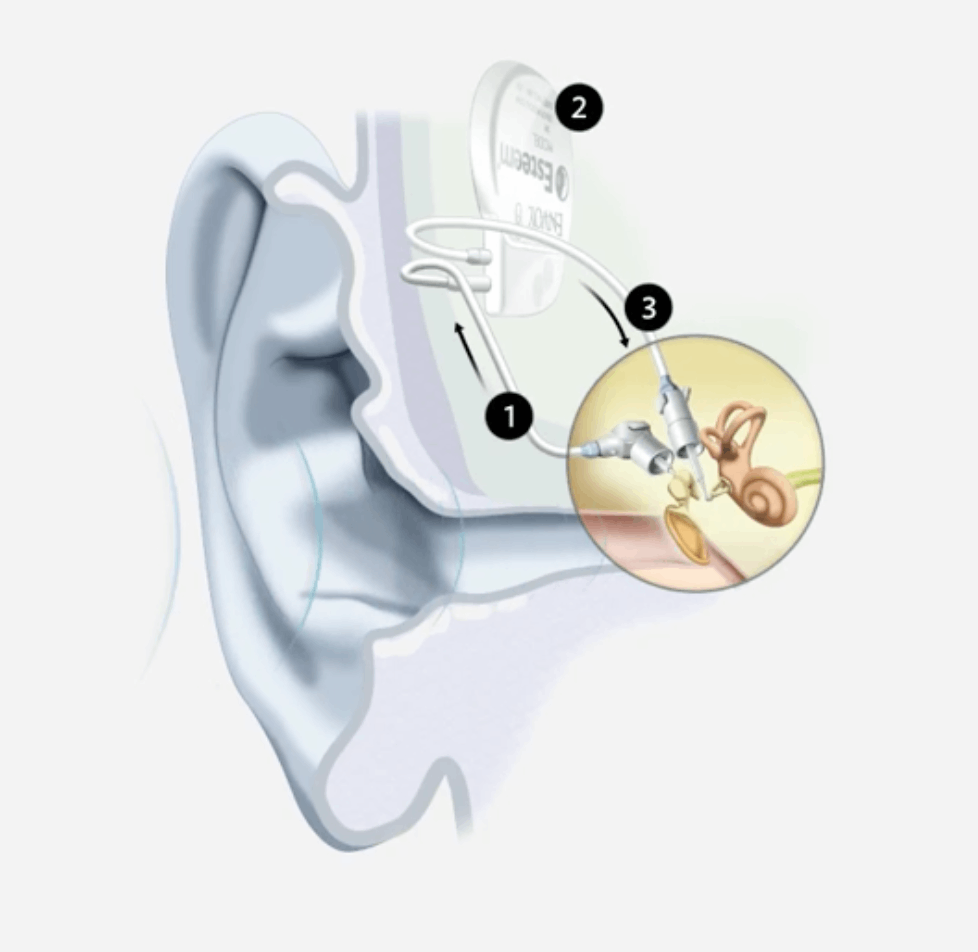
Key features of the Esteem implant include:
Envoy Sensor, which converts vibrations into electrical signals that are sent to the implanted Esteem Sound Processor.
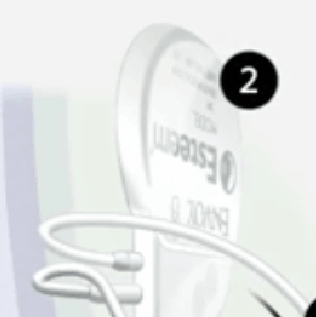
Esteem Sound Processor, which receives, adjusts and intensifies the signals to fit individual hearing needs.
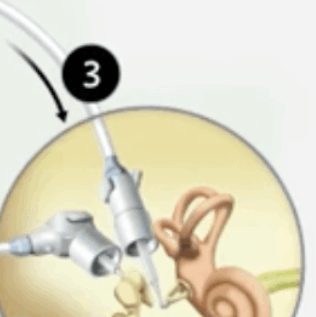
Envoy Driver, which directly transfers these signals to the inner ear where the hair cells are stimulated, causing hearing.
For illustrative purposes only; may not be indicative of individual characteristics, size placement, functionality, or use.
Read about the Esteem implant surgical procedure and important safety information here.
Fully Implanted Acclaim® Cochlear Implant* Pivotal Study Sites in Top U.S. Cochlear Implant Centers
The Fully Implanted Acclaim® Cochlear Implant* clinical study sites have staff, capacity, training, and experience on both the surgery and audiology side to support successful commercialization. Strategy is for these sites to become the backbone of the Fully Implanted Acclaim® Cochlear Implant* commercial launch.
Four of these sites are also affiliated with medical schools and residency and fellowship programs for otologists and neurotologists:
- Mayo Clinic, Rochester, MN
- Cleveland Clinic, Cleveland, OH
- Community Hospital/Hearts for Hearing, Oklahoma City, OK
- University of Florida, Gainesville, FL
- Medical University of South Carolina, Charleston, SC
- Shohet Ear Associates, Seal Beach, CA
- Center for Neurosciences, Tucson, AZ
JOIN US:
Envoy Medical Leadership

Brent T. Lucas
CEO

Chuck Brynelsen
Chairman of the Envoy Medical Board

Glen Taylor
Chairman Emeritus of the Envoy Medical Board, Largest Shareholder, Founder/Chairman of Taylor Corporation & The Minnesota Timberwolves
Envoy Medical News

New CPT Codes for Totally Implantable Active Middle Ear Hearing Implants Go into Effect July 1, Unlocking New Opportunities for Envoy Medical
June 26, 2025

Envoy Medical’s Pivotal Clinical Trial for Fully Implanted Acclaim® Cochlear Implant Remains On Track After First Month Follow-Up
June 10, 2025



